
Are you getting the whole story?

Molecular Subtyping provides clinicians a “genetic view” of their patient’s breast cancer.
Traditional methods to classify breast cancer subtypes using IHC may provide a view from the receptor level, but a deeper look at the downstream signaling may present a different story.
Uncovering the hidden ER+ Basal-type patient.
A recent publication in npj | Breast Cancer, describes how traditional “luminal” patients (ER+, HER2- by IHC) are reclassified by BluePrint® Molecular Subtyping as “Basal-type”, with clinical outcome and response to neoadjuvant chemotherapy similar to “triple negative” patients (ER/PR-, HER2-).
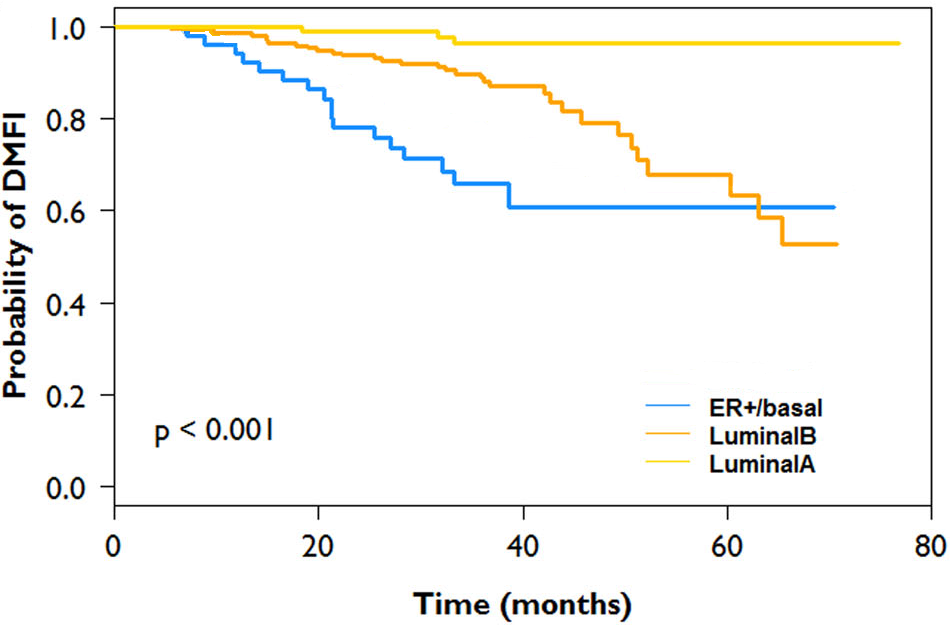
Compared with Luminal A and B patients that have a 3-yr Distant Metastasis Free Interval (DMFI) of 96% and 89% respectively, the ER+ Basal-type patients have a 3-year DMFI of 66%.
Coupled with a pathologic complete response rate of 34% with pre-operative chemotherapy (compared to 2% for Luminal A and 6% for Luminal B patients) with a significantly better survival for patients that achieved pCR vs those with residual disease (78% vs 61%, p<0.001), the ER+ Basal-type patients are in need of more than just endocrine therapy alone.
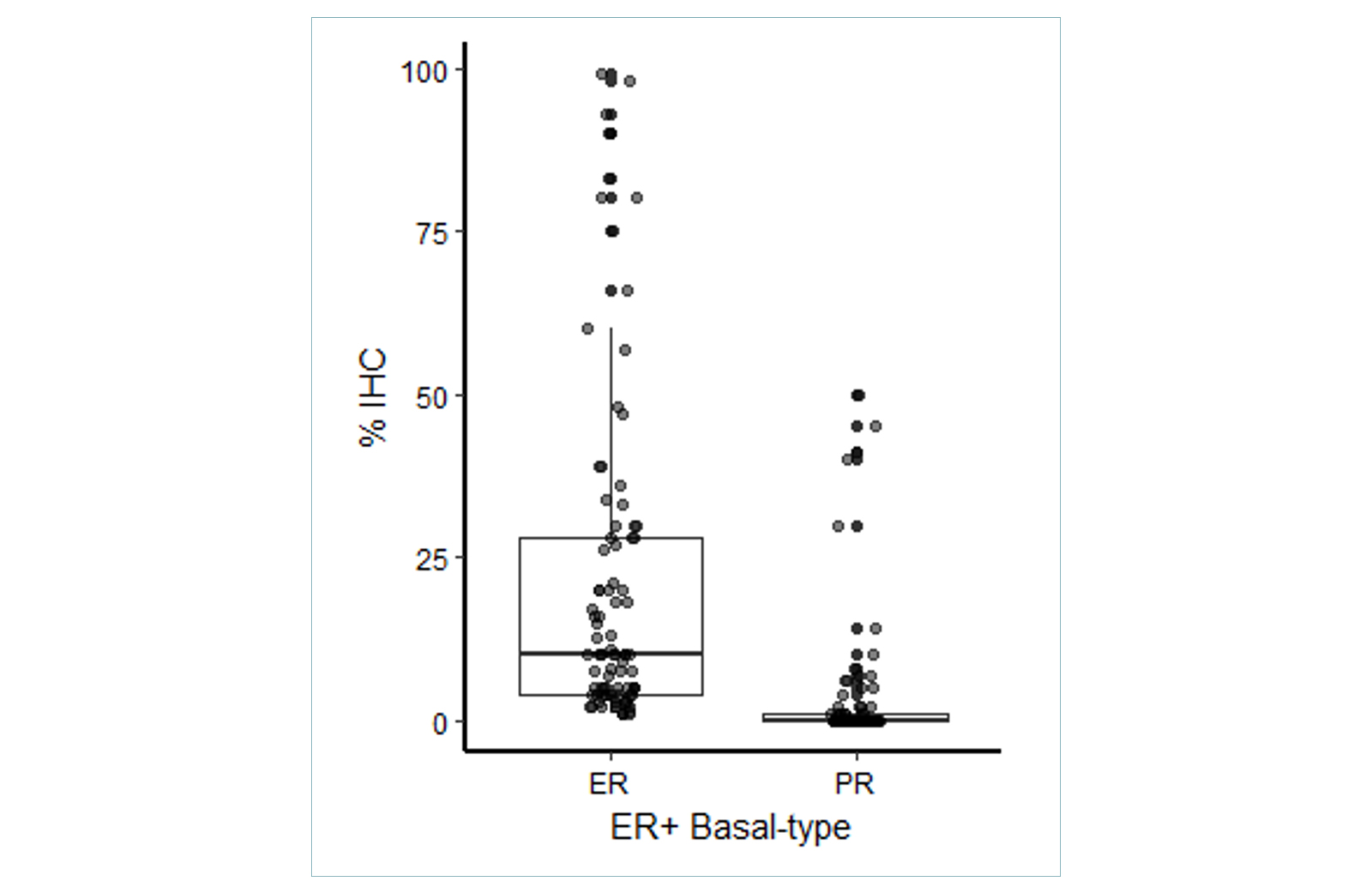
In ER+ Basal-type tumors, there is a broad spectrum of ER and PR positivity detected by IHC. These data demonstrate that the ER+ Basal phenotype is not only restricted to patients with low ER/PR expression.
Want more detail?
Read our article in npj | Breast Cancer.

Reference: Groenendijk, F., et al. NPJ Breast Cancer. 2019 Apr 18;5:15

BluePrint Molecular Subtyping is an 80-gene test that classifies breast cancer into one of three molecular subtypes; Luminal-type, HER2-type, or Basal-type. Each subtype has a unique profile of response to neoadjuvant chemotherapy, targeted therapy and long-term outcome, which can help clinicians refine their treatment strategy for breast cancer patients.
